 In our January post on what we planned to watch for in 2018, we predicted that digital health would come to the foreground of activity this year. And indeed, we are starting to see a lot of buzz around digital health. In March, Novartis and Pear Therapeutics announced the first development deal between a major pharmaceutical company and a digital therapeutics firm. The collaboration, which combines Novartis’ expertise in biomedical research and clinical development with Pear’s expertise in digital therapeutics, seeks to develop new products for patients with schizophrenia and multiple sclerosis that better address the full burden of such diseases.
In our January post on what we planned to watch for in 2018, we predicted that digital health would come to the foreground of activity this year. And indeed, we are starting to see a lot of buzz around digital health. In March, Novartis and Pear Therapeutics announced the first development deal between a major pharmaceutical company and a digital therapeutics firm. The collaboration, which combines Novartis’ expertise in biomedical research and clinical development with Pear’s expertise in digital therapeutics, seeks to develop new products for patients with schizophrenia and multiple sclerosis that better address the full burden of such diseases.
The Pear THRIVE™ digital therapeutic for schizophrenia is already in clinical development, having demonstrated potential usability, patient retention, and preliminary efficacy in three clinical trials involving more than 1,000 patients. THRIVE delivers clinically proven treatments like cognitive behavioral therapy through a mobile or desktop app, and allows patients to access medical care at their convenience rather than journeying to a session with a therapist. Once the app gains regulatory approval, the intention is to prescribe its use alongside drug therapy to help patients manage the symptoms of schizophrenia, including hallucinations and delusions, and to provide support for schizophrenia-related mood disorders.
The planned digital therapeutic for multiple sclerosis will be designed to address the considerable mental health burden of depression, anxiety, fatigue, and cognitive impairment faced by patients with this disease.
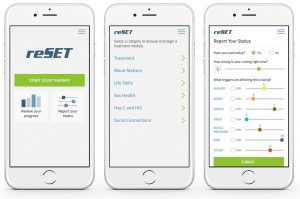 Pear has already achieved a significant milestone in the world of digital therapeutics, as they were the first to receive FDA clearance for a therapeutic app. In September 2017, Pear received FDA marketing clearance for the first prescription digital therapeutic to treat a disease, with their reSET® app for the treatment of patients with substance abuse disorder. Novartis has also signaled its commitment to the field of digital therapeutics, hiring a new chief digital officer and investing in software designed to enable clinical trials to be carried out in patients’ homes. This latter move could also help the company’s clinical trials in other areas by helping Novartis reach a greater diversity of patients as compared to traditional study visits at academic or other large hospital settings.
Pear has already achieved a significant milestone in the world of digital therapeutics, as they were the first to receive FDA clearance for a therapeutic app. In September 2017, Pear received FDA marketing clearance for the first prescription digital therapeutic to treat a disease, with their reSET® app for the treatment of patients with substance abuse disorder. Novartis has also signaled its commitment to the field of digital therapeutics, hiring a new chief digital officer and investing in software designed to enable clinical trials to be carried out in patients’ homes. This latter move could also help the company’s clinical trials in other areas by helping Novartis reach a greater diversity of patients as compared to traditional study visits at academic or other large hospital settings.
A number of other drug companies have also made investments in digital startups, working in areas of therapeutic gaming, smartphone apps, and sensors buried in pills or attached to medical dispensers.
We expect the field of digital therapeutics and medical apps will grow even faster in the future as the FDA is providing important assistance in the form of new guidelines (still in development) that will help companies understand how to prove the safety and effectiveness of such apps. And while reimbursement is still limited, we can see the situation beginning to evolve. Medicare recently extended coverage to pre-diabetes preventive services that are not yet in digital form, but could soon be, which could greatly expand access to care.