Cancer immunotherapy has improved outcomes in many patients suffering from hard-to-treat cancers. However, the same treatments that benefit some patients seemingly lead to minimal-to-no response in others. A growing understanding of cancer biology increasingly points to the complex role of the immune microenvironment in influencing cancer outcomes. The functions, locations, and characteristics of various immune cells within and around tumors appear to shape the microenvironment in ways that enable cancer cells to disturb or thwart immune attacks, develop resistance to treatment, facilitate metastasis, and further support tumor growth and survival in other ways. Researchers are now looking to map and describe the workings of the tumor immune environment to find ways to potentiate an immune response or counteract immunosuppressive signals, in hopes of reprogramming cancer-promoting immune cells to attack the tumor rather than promoting its survival. Here, we look at some of the latest research breakthroughs in the field.
Tumor immune environment mapping
A greater understanding of the tumor immune environment and its impact on gene and protein expression is helping researchers uncover key pathways and molecular targets across cancer types, with the potential to develop new tailored treatment strategies.
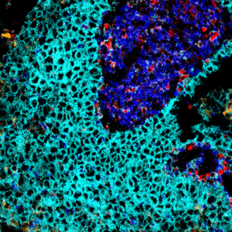
For example, researchers at the Ludwig Cancer Research Institute analyzed 100 brain tumor samples of differing types (primary, metastatic, low-grade, high-grade, gliomas, etc.) to profile the numbers and locations of 14 different types of immune cells within the tumors. They also further characterized the full spectrum of proteins and gene expression patterns of individual immune cells within each tumor sample. The researchers then used these detailed analyses to map the immune state of each tumor type and identify functional differences in the resident immune cells. They found that five types of cells were mainly responsible for shaping the brain tumor microenvironment: monocyte-derived macrophages that infiltrated the brain from other areas of the body; microglia, the brain’s resident version of those cells; neutrophils; CD4+ T cells; and CD8+ (killer) T cells. Their analysis showed that the specific composition of the immune environment and the activation state of the cells within varied greatly by tumor type, suggesting that brain cancer is not just “one condition” and that different treatment strategies might be more effective towards different subtypes. For example, brain metastases of melanoma — one of the few brain tumors that have responded to checkpoint inhibitors – were found to have an abundance of T cells. Gliomas, however, had almost none but were rich in macrophages and microglia. Thus, an approach that activates existing T-cells might be effective in the melanoma brain metastases while an approach that increases T-cell infiltration might offer more promise for gliomas.
In lung cancer, Johnson & Johnson (J&J) and Boston University researchers have found genomic alterations in immune cells within precancerous lung lesions that might serve as early biomarkers of invasive tumors. The researchers took bronchial biopsies and brushings from high-risk smokers with precancerous lesions over several years to monitor whether the lesions progressed to lung cancer. They grouped the biopsies into four genomic subtypes – proliferative, inflammatory, secretory and normal-like – and found that the proliferative subtype showed significant suppression of genes coding for proteins involved in the immune response. These observations point to a possibly impaired immune environment and suggest that immune-modulating treatments might be effective in delaying or reversing lung cancer’s development. The same molecular alterations the researchers found in precancerous lesions can also occur in normal upper airway cells, suggesting that it may be possible to identify individuals at high risk of lung cancer using endoscopic tests that are less invasive than lung biopsies.
Immune response potentiation
A number of promising research studies have been published in recent months suggesting new strategies for fighting cancer based on potentiation of the immune response.
For example, researchers at the Icahn School of Medicine at Mount Sinai found that injecting immune stimulants directly into a lymphoma tumor site can instruct T-cells to kill the tumor cells and help patients fight off their cancer. Separately, a preclinical study by researchers at the University of Louisville, Kentucky showed that injection of the protein SA-4-1BBL could activate CD4+ T-cells and NK cells, and prevent healthy mice from developing cervical and lung cancer.
B-cells are adaptive, and in addition to producing antibodies, they mediate T-cell activation, including within tumors. Recent research suggests that the presence of B cells within the tumor microenvironment could potentially serve as a predictive marker of a patient’s response to immunotherapy. Researchers from M.D. Anderson Cancer Center and Lund University found that when B-cells are present in some melanoma tumors, they localize within structures called tertiary lymphoid structures (TLS); tumors with TLS have shown improved patient survival and response to immunotherapy. Conversely, in tumors lacking TLS, B-cells appeared to be dysfunctional and patient response to immunotherapy limited.
Counteracting immunosuppressive signals
Macrophages play an important role in promoting a tumor environment that suppresses T-cell activity, in part through the expression of the TREM2 receptor at their surface. TREM2 is known to promote an immunosuppressive environment that can limit the efficacy of immune-activating treatments like checkpoint inhibitors, and high levels of TREM2 within tumors have been found to correlate with shorter survival in colorectal and breast cancers. Researchers at Washington University School of Medicine recently demonstrated that they could enhance checkpoint inhibitor effectiveness in mice bearing sarcoma tumors by administering the immunotherapy along with an anti-TREM2 antibody. In animals receiving both therapeutics, the researchers found that immunosuppressive macrophages were absent, while present T-cells were active against the cancer, resulting in complete elimination of the tumors. Given that TREM2 is expressed at high levels by macrophages within tumors and not outside, the research findings suggest anti-TREM2 agents could potentially aid checkpoint inhibitor activity while having little effect on peripheral tissues.
Several additional ongoing studies are looking at the role of the immune microenvironment in tumor response to treatment and how it can be best leveraged to enhance cancer therapy effectiveness.