The COVID-19 pandemic has caused many disruptions in pharmaceutical development, forcing companies to increasingly adopt technologies and practices that support the conduct of decentralized clinical trials. Government regulators have also adjusted their own policies and practices to support trial design changes in ways that accommodate such remote trials while still ensuring the rigor of those studies.
We recently examined some of the factors that have led to the successful use of such studies and discussed how these trial designs represent an opportunity for greater agility in clinical development. This is especially true for cases where the nature of the disease or patient type to be enrolled can add significant time and cost to a traditional trial design.
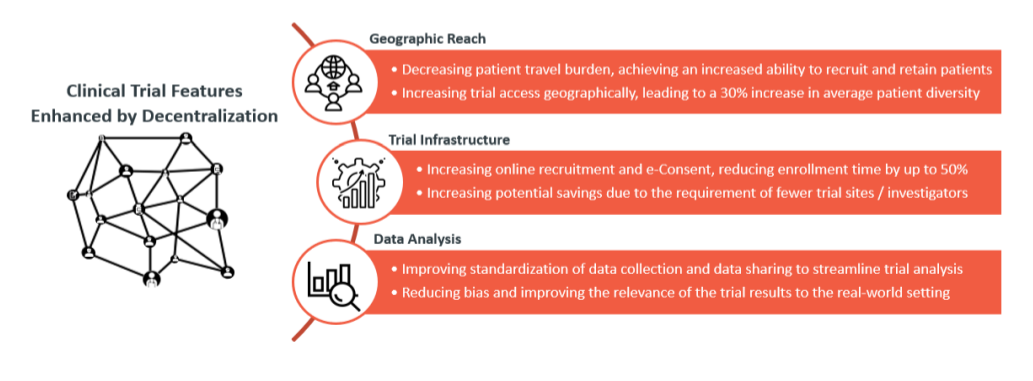
The result is our new white paper, “Next-Gen Clinical Development: Why Companies Should Invest in Remote Clinical Trials,” which discusses which studies may especially benefit from a decentralized approach and the particular trial features that could be enhanced.
One long recognized challenge that could take advantage of the growing shift toward decentralized trials is that of increasing diversity amongst the participants enrolled. Populations often under-represented in clinical trials — including older adults, pregnant women, children, and racial / ethnic minorities — can be affected by health conditions and respond to treatments in different ways. Indeed, a review by the U.S. Food and Drug Administration of 167 new molecular entities found that 1 in 5 of the examined drugs showed differences in patient exposure and response that cut across racial and/or ethnic groups.
And yet, according to a JAMA Network Open Study from February 2021 that looked at vaccine trials completed between July 2011 and June 2020, diversity in clinical trials remains substantially lacking. The JAMA analysis found that more than 77% of clinical trial participants were White, with Hispanic/LatinX, Black/African American, Asian and Native American/Indigenous Alaskan participants significantly under-represented in each of the examined trials. Other studies have shown that people over age 65 are also typically not well represented in clinical trials. Moreover, while overall trial participation by women has been on the rise, significant gaps remain, such as in cardiology studies, despite the acknowledgement that women’s outcomes in heart disease are often very different from those for men. Thus, a critical need exists to include more diverse populations in clinical trials to better understand the potential risks and benefits of new medicines across populations, to provide more accurate and comprehensive data sets, and to help foster a broader acceptance of new interventions among hesitant populations.
Several factors have contributed to this lack of diversity in clinical trials. Some are logistical or cultural, including a patient’s location, language and cultural barriers, as well as time and resource constraints, all of which can increase the burden of participation for patients and caregivers. Lack of awareness of a trial by patients and their physicians, and patient mistrust/ lack of comfort with the trial process further contribute to the problem. Other factors limiting diversity relate to the need for focused trials that offer the highest probability of success for speeding a new drug or vaccine to market. Enrolling a broader population can add significant recruitment time and overall cost to a trial, and potentially open the door to more adverse events, adding risk to a drug’s ultimate success.
The move towards more decentralized clinical trials spurred by the pandemic has been enabled by the development and increasing use of remote monitoring technologies, telehealth, in-home diagnostic testing, and other new tools and strategies. In addition to encouraging companies to make clinical trial design smarter and implementation cheaper and faster, this shift is also offering valuable new opportunities to improve diversity among those enrolled.
Site-less trials can open up study participation to more regions and different patient populations, making recruitment easier by significantly decreasing the time, cost, and other travel burdens for trial participants and their families. The use of home-based trial solutions–such as home nurse visits, virtual appointments and at-home lab tests–are increasingly being incorporated in decentralized trials. These tools and strategies further reduce stress for trial participants, lower potential health risks associated with travel to trial sites, and ease the burden of participation for patients, especially for the elderly or those affected by degenerative conditions. Moreover, the increasing involvement of community-based medical staff in decentralized trials can help increase both greater awareness of clinical studies and trust among the potential participants.
Diversity and Inclusion as Corporate Priorities
Recognizing the need for change, a growing number of pharmaceutical and biotechnology companies are now making concerted efforts to incorporate more diversity in their trials and throughout their businesses. Seven out of 10 biotech companies now list diversity and inclusion in their value statements as a corporate priority, an increase from 46% in 2019, and many are taking specific actions to address the issue.
During the Summer of 2020, for example, Lilly and the National Institutes for Allergy and Infectious Disease (NIAID) deployed a fleet of specialized mobile units to support Lilly’s COVID drug trials at long-term care facilities, whose residents were most vulnerable to the pandemic’s risks. This year, Lilly is building on that effort to add greater diversity to its studies with a new fleet of 20 recreational vehicles that will help bring its clinical trials to potential participants in predominantly Hispanic and Black areas and lower-income rural regions.
Novartis launched an ambitious 10-year initiative in July to create a new generation of clinical researchers, data experts and physicians who can help build greater diversity into their drug development programs. The company has instituted a $20 million Thurgood Marshall College Fund to support training and research programs at Morehouse School of Medicine and 26 other historically black colleges and universities. An additional $13.7 million will establish three research centers for clinical trials aimed at better understanding the health effects of environmental issues and climate change on different populations and for projects aimed at addressing health equity issues. Novartis plans to reach out to other companies to join its program for even greater impact.
Similarly, Genentech has formed a new coalition of clinical research sites aimed at increasing diversity in the company’s oncology studies, partnering with centers in regions with higher Black and Hispanic/LatinX populations.
The FDA is also supporting the push to increase diversity in clinical trials. In November 2020, the agency issued its final guidance on designing and executing clinical studies of drugs and biologics to include people with different demographic (sex, race, age, location) and non-demographic (patients with organ dysfunction, co-morbidities, disabilities, weight range extremes, and populations with rarer diseases or conditions) characteristics. The guidance provides drug developers with the agency’s thinking on steps that could be taken to broaden eligibility and increase enrollment of under-represented populations in clinical trials through inclusive recruitment practices, trial design, and methodological approaches.