
In 2018, French President Emmanuel Macron had proposed the formation of a Pan-European Union innovation agency similar to DARPA, the decades-old U.S. Department of Defense’s Advanced Research Project Agency that supports the development of emerging, high-risk/high reward technologies for military use. While the European Commission said that they shared President Macron’s concerns about the sparsity of job-creating innovation in Europe at that time, they rejected the idea of a European DARPA as being too “top-down” and too military-focused. Rather than following DARPA’s model of calling for and funding research in areas of specific interest, as well as directly purchasing innovative products and technologies, the Commission felt they simply ought to support emerging companies and give advice on the scale-up of promising technologies. Moreover, some members of the Commission voiced hesitancy over forming a new EU-level agency because previous attempts had received lackluster support primarily due to budgetary constraints.
However, lessons learned in the early stages of the COVID-19 pandemic have changed the EU’s thinking around creating an agency to provide DARPA-like anticipatory support for innovation, at least in the field of healthcare and cross-border health crisis response. The first few months of the pandemic had moved public authorities at the EU-wide, national and regional levels to react and collaborate in new ways. However, they reacted to events on an ad hoc basis without much overall management or pre-emptive activity. As a result, delays and shortages in procuring and distributing vaccines and other medical supplies to fight the spreading coronavirus infection were commonly seen in many EU regions. In contrast, the United States was able to build on DARPA’s decade of pre-pandemic funding for nucleic acid vaccine research (both mRNA and DNA vaccines), which the agency saw as a strategy for faster and lower cost vaccine development and production compared to traditional methods. For instance, some of Moderna’s earliest funding for the mRNA platform, which was used to create their COVID-19 vaccine, came from DARPA in 2013. Other companies that quickly developed vaccines, diagnostics, and therapeutics against the virus also benefited from DARPA funding prior to and/or during the pandemic, including AbCellera Biologics, CureVac, Inovio Pharmaceuticals, Regeneron, and Vir Biotechnology.
In February 2021, the European Commission launched the European Health Emergency Preparedness and Response Authority (HERA), initially to detect and analyze new variants of the COVID-19 virus and to develop and produce vaccines adapted to those viruses. By June 2021, the Commission had broadened HERA’s mandate, based on a more DARPA-like model, to ensure that the European Union and member states could more readily and quickly act on multiple levels in the face of another cross-border health crisis. To support the new Agency’s activities, the Commission dedicated funding of approximately 30 billion Euros – 6 billion Euros over the next five years from the EU Multiannual Financial Framework and 24 billion Euros from a variety of other EU programs along with private funding. HERA issued its first workplan in February 2022.
HERA’s overarching mission is to arm and support member states in both readiness and emergency response. In its normal ‘Preparedness’ mode, the agency is involved in threat assessment and intelligence gathering, giving support to advanced research and development for medical countermeasures (diagnostics, vaccines, antibodies, drugs, medical supplies and personal protection equipment), strengthening medical knowledge and skills, boosting manufacturing capacity, increasing stockpiles, and facilitating procurement and distribution.
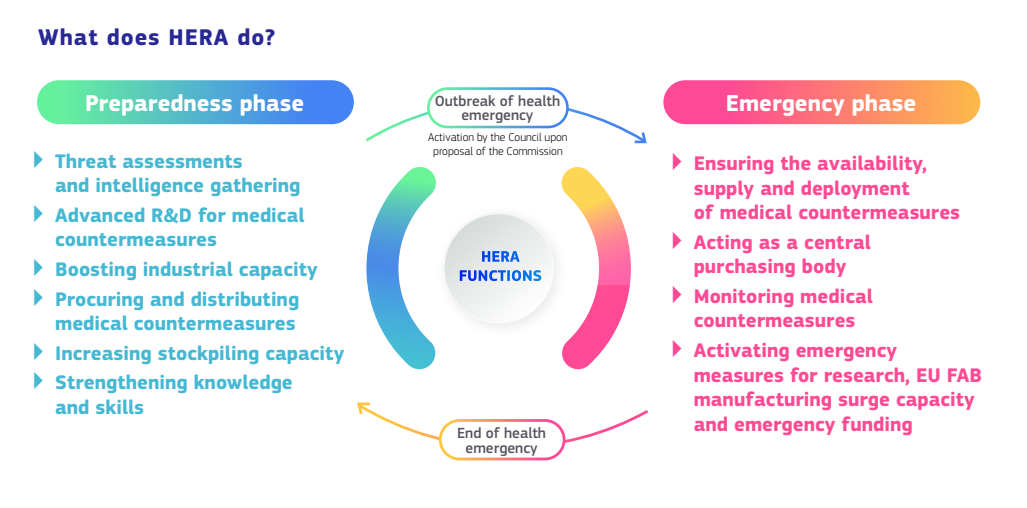
The Agency’s ‘Emergency Response’ mode can be activated in response to a serious cross-border health threat by the HERA Crisis board, whose members include one representative from each EU state, along with the President of the European Union, the EU Commissioner for Health and Food Safety, and other Commission members as appropriate. In its Emergency Response role, HERA will act as a central purchasing body, ensuring and monitoring the equitable supply and deployment of medical countermeasures, and activating crisis measures for research, manufacturing surge capacity and emergency funding. HERA will also lead EU collaborations with global partners to address supply bottlenecks and address global disease surveillance, as well as support the ability of low and middle income countries to build expertise and develop manufacturing and distribution capabilities. HERA is further advised by a Joint Industrial Cooperation Forum, composed of company representatives and European Commission members who will offer HERA industry feedback on capacities and bottlenecks.
HERA’s objectives for 2022 include working with other EU and national health agencies, industry, the research community, regional organizations and international partners to support research and development; to establish a large-scale EU platform for clinical trials and data sharing; and to identify three high-impact health threats beyond COVID-19. In addition, the agency is building an IT platform and intelligence network for threat assessment and prioritization as well as developing a network of “ever-warm” manufacturing facilities that can be quickly mobilized in an emergency.
While DARPA was clearly instrumental in the U.S. response to the pandemic, experts have deemed that agency inadequate to address many critical health-related needs in the United States. DARPA is designed to serve the needs of a single customer – the U.S. military – and the Agency is focused primarily on national security needs and programs with an engineering focus. In contrast, other important health care problems are not well addressed by engineering solutions, as they involve complex biological systems, human behaviors, and environmental and social factors. Moreover, many major health problems impact a wider variety of stakeholders than the military and national security agencies, including patients, healthcare providers, payers and policy-makers. Thus, in March 2022, President Joe Biden authorized the establishment of the Advanced Research Projects Agency for Health (ARPA-H), an agency modeled on DARPA, whose aim will be to support transformative high-risk/high-reward research to drive biomedical and health breakthroughs beyond those with military applications. In addition, the President has called for $6.5 billion in funding over ARPA-H’s first three years, with priorities to be directed to HIV/AIDS and other infectious diseases, cancer, Alzheimer’s disease, diabetes and other chronic conditions, and projects related to drug misuse and addiction, opioid alternatives, and other pain management strategies.