As more gene and cell therapies come to market, a big challenge facing both drug developers and payers is designing and instituting appropriate pricing models for these one-time, potentially curative treatments. Innovation should be rewarded, and a curative — or at least, very long-lasting — treatment is likely to save significant dollars in the typical costs of patient care over time. At the same time, major concerns exist as to how healthcare systems can absorb dealing with the coming wave of such high-priced new medicines. As of January 2019, approximately 90 one-time therapies were in Phase 3 development, several of which are expected to reach the market over the next few years.
 During the JP Morgan Healthcare Conference, Bluebird Bio revealed a potential outcomes-based pricing scheme for Lentiglobin BB305, the company’s gene therapy for transfusion-dependent beta-thalassemia. Under the company’s proposed pay-by-installment plan, payers would make up to five equal outlays for the therapy over five years, with each payment beyond the first one contingent on the continued success of the treatment. While Bluebird has not yet disclosed the planned price for Lentiglobin B3305, the company’s Chief Executive Officer said it would not exceed $2.1 million or increase above the consumer price index. The goal of Bluebird’s gene therapy is to reduce the patient’s need for blood transfusions and to avoid health complications that are sometimes caused by those transfusions. Current treatments for the disease, under traditional pricing methods, typically cost $1.5 to 3 million over five years.
During the JP Morgan Healthcare Conference, Bluebird Bio revealed a potential outcomes-based pricing scheme for Lentiglobin BB305, the company’s gene therapy for transfusion-dependent beta-thalassemia. Under the company’s proposed pay-by-installment plan, payers would make up to five equal outlays for the therapy over five years, with each payment beyond the first one contingent on the continued success of the treatment. While Bluebird has not yet disclosed the planned price for Lentiglobin B3305, the company’s Chief Executive Officer said it would not exceed $2.1 million or increase above the consumer price index. The goal of Bluebird’s gene therapy is to reduce the patient’s need for blood transfusions and to avoid health complications that are sometimes caused by those transfusions. Current treatments for the disease, under traditional pricing methods, typically cost $1.5 to 3 million over five years.
Bluebird’s proposed pricing strategy was reportedly well-received by some payers who felt its multi-year framework would help to limit the upfront budget impact of the therapy while addressing the current lack of long-term data on its efficacy. The model is potentially also flexible enough to allow contract negotiations or mutual recognition strategies between payers to deal with payment issues in cases where a patient switches insurer before the final payment is due. The workability of an installment-based payment plan outside of the United States is less certain, however, as some countries’ single-payer models may not allow for such a pricing structure.
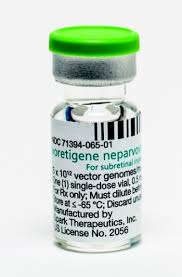 Until recently, most attempts to develop value-based pricing in the United States have been around chronic care, with pricing contracts with commercial insurance plans linking reimbursement to patient outcomes. But, increasingly, such strategies are being proposed for rarer diseases. For example, pricing for Spark Therapeutics’ Luxturna gene therapy, which treats a form of inherited blindness, runs approximately $425,000 per eye, and the company has offered retroactive rebates to some insurers if the treatment doesn’t work as designed.
Until recently, most attempts to develop value-based pricing in the United States have been around chronic care, with pricing contracts with commercial insurance plans linking reimbursement to patient outcomes. But, increasingly, such strategies are being proposed for rarer diseases. For example, pricing for Spark Therapeutics’ Luxturna gene therapy, which treats a form of inherited blindness, runs approximately $425,000 per eye, and the company has offered retroactive rebates to some insurers if the treatment doesn’t work as designed.
A few governmental payers are instituting their own innovations in financing drugs for their Medicaid or incarcerated patients, with a Netflix-like subscription model for curative but costly Hepatitis C medications. Under this model, the government pays installments over a given period of time in exchange for unlimited treatment access, enabling them to offer low or no co-payments to patients.
Given the number of one-and-one done therapies that will likely reach the market in the coming years, can any of these newer pricing strategies be sustainable for the U.S. healthcare system? If not, what further pricing innovations should be proposed and what are their prospects for success in balancing innovation, access, and affordability?