 Digital technologies are becoming an increasingly important part of modern healthcare. Medical devices have the ability to connect to and communicate with other devices or systems. Not only are new types of devices being created, but many existing devices are being updated with digital capabilities. As a result, the U.S. Food and Drug Administration (FDA) has made digital health a major area of focus and policy changes within the medical device field, aimed at bringing efficiency and modernization to their regulatory oversight of such products with a goal of encouraging innovation and the development of safer, more effective medical devices.
Digital technologies are becoming an increasingly important part of modern healthcare. Medical devices have the ability to connect to and communicate with other devices or systems. Not only are new types of devices being created, but many existing devices are being updated with digital capabilities. As a result, the U.S. Food and Drug Administration (FDA) has made digital health a major area of focus and policy changes within the medical device field, aimed at bringing efficiency and modernization to their regulatory oversight of such products with a goal of encouraging innovation and the development of safer, more effective medical devices.
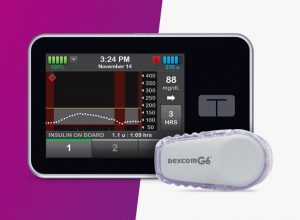
In line with these goals, the FDA granted an approval to Tandem Diabetes Care in February for the first truly interoperable insulin pump. In doing so, the agency also established a new medical device classification for such pumps, called Alternate Controller-Enabled Infusion Pumps (ACE pumps) and “special controls” that outline regulatory requirements for assuring the “accuracy, reliability, cybersecurity, and clinical relevance” of similar devices, along with the types of studies and data requirement to demonstrate acceptable performance.
The new t:slim X2™ insulin pump gives diabetes patients the ability to customize their own diabetes management systems by digitally linking their preferred devices — automated dosing systems, continuous glucose monitors, and blood sugar meters — to the new pump. The new pump offers a large touchscreen, rechargeable battery, Bluetooth wireless technology, USB connectivity, and the ability to update software remotely through a personal computer, enabling users to quickly access new features of the pump once they are approved. It is designed to securely and reliably communicate with any compatible external device to receive, execute and confirm drug delivery commands. The diabetes community has applauded the FDA’s move, hailing it as an important step towards creating a truly interoperable plug-and-play ecosystem where people with diabetes can better tailor their own approach to care.
The new device classification gives manufacturers more flexibility for making improvements in such devices and enables a more predictable and efficient pathway for the development and marketing clearance of new systems. Importantly, the new classification separates FDA oversight of the software from that of the device hardware, enabling software upgrades to be reviewed and approved by the agency without the need for the device to undergo testing and regulatory review with each change. Additionally, future interoperable pump developers adhering to these guidelines will be able to seek approval for their devices through a 510K clearance, a regulatory pathway that is generally faster and less onerous than a de novo approval. Overall, this new FDA policy is expected to allow for faster innovation, and ultimately, better pumps for patients.
Will other developers of devices for diabetes management follow suit and ensure their own products can “play nicely” with these new pumps? What other types of medical devices might be appropriate for similar new device classifications and regulatory policy changes by FDA? We’ll be watching.