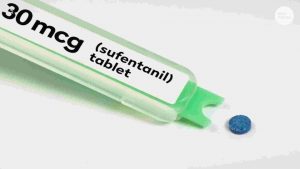
 Does the United States really need another opioid drug, given the enormous public health crisis of opioid addiction facing the country today? Many experts are asking this question about the FDA’s approval in November of Dsuvia (sufentinil), a powerful opioid ten times stronger than fentanyl, the drug responsible for many opioid overdose deaths in recent years.
Does the United States really need another opioid drug, given the enormous public health crisis of opioid addiction facing the country today? Many experts are asking this question about the FDA’s approval in November of Dsuvia (sufentinil), a powerful opioid ten times stronger than fentanyl, the drug responsible for many opioid overdose deaths in recent years.
The FDA’s Anesthetic and Analgesic Drug Products Advisory Committee (AADPAC) voted 10:3 in favor of the drug’s approval in October, despite serious questions about the drug’s safety and efficacy compared to morphine. However, AADPAC Chair Raeford Brown, M.D., who was not present for the vote, co-wrote a letter with a group of health experts at the consumer advocacy organization Public Citizen begging the FDA not to approve the drug.
In response, FDA Commissioner Scott Gottlieb made a rare public statement to explain the agency’s approval of Dsuvia, a sublingual (under the tongue) tablet, as an alternative therapy for use in settings where intravenous administration of opioid drugs is not possible and for patients in need of powerful pain relief who cannot swallow a pill.
Dr. Brown, Professor of Anesthesiology and Pediatrics at The University of Kentucky and The Kentucky Children’s Hospital and Chair of the Section on Anesthesiology and Pain Medicine at The American Academy of Pediatrics, questioned the need for another, more powerful opioid given that some 440 strong analgesic formulations are currently available. This includes Abstral, a form of fentanyl that is also administered sublingually and indicated for the treatment of breakthrough cancer pain.
The FDA acknowledges the considerable risks of Dsuvia, and has mandated a stringent risk-management plan to control its use. The drug will not be available through retail pharmacies for patients to take home, but instead must be administered by a healthcare professional in a medically supervised setting. However, some experts wonder whether such controls are sufficient given that fentanyl and its analogs — which were also placed under strict controls — have found their way onto the street.
Will the Risk Evaluation and Mitigation Strategy mandated by FDA for this drug be sufficient to block its abuse and prevent it from further compounding drug overdose problems?