 We have written previously about the need for earlier diagnosis for Alzheimer’s disease (AD) and other dementias, a capability that could both aid in the discovery and the development of new therapies, and increase the chances for better patient outcomes in slowing or preventing cognitive decline. Recently, researchers have presented data on several new non-invasive diagnostic approaches that have the potential to identify those who are likely to develop dementia as early as 7-10 years before conventional methods used today.
We have written previously about the need for earlier diagnosis for Alzheimer’s disease (AD) and other dementias, a capability that could both aid in the discovery and the development of new therapies, and increase the chances for better patient outcomes in slowing or preventing cognitive decline. Recently, researchers have presented data on several new non-invasive diagnostic approaches that have the potential to identify those who are likely to develop dementia as early as 7-10 years before conventional methods used today.
Two of these new methods focus on key changes in blood vessels and may offer non-invasive and relatively inexpensive ways to diagnose AD or predict greater risk of dementia. The first is a five-minute ultrasound scan that analyzes pulse intensity in the blood vessels of the neck. This method may be able to identify people at risk of dementia up to 10 years before symptoms emerge. The study included 3,200 patients aged 58 – 74 who underwent ultrasounds of their neck in 2002, and then had their cognitive function monitored for up to 14 years. Individuals with the most intense neck pulses were found to be up to 50% more likely to suffer reduced cognitive function over time. The reason: the more intense pulses reflect a greater difficulty of pumping blood into the brain, with the greater pumping intensity leading to arterial damage and reduced blood flow over time. Vascular dementia is caused by reduced blood flow to the brain, which also plays a key role in the development of AD; these two conditions account for the majority of dementia cases. If these initial data are confirmed, they may also point to strategies for lowering risk of dementia through therapeutic interventions that improve vascular and heart health overall.
Changes in the small blood vessels of the eye also appear to provide a clue to the early diagnosis of AD. Two recent studies each used a five-minute, light-based scan of a cross-section of the retina using optical coherence tomography, a technology commonly used to guide treatments for diseases affecting the retina and optic nerve, such as glaucoma, age-related macular degeneration, and diabetic eye diseases. The researchers found that AD patients had lost blood vessels at the back of the eye and noted that a specific layer of the retina was thinner. They could detect alterations in patients with AD, including those with no family history of the condition, and could distinguish between those with AD and those with mild cognitive impairment. They speculated that deterioration of the retina in AD patients may mirror disease progression in the brain, conveyed across the optic nerve.
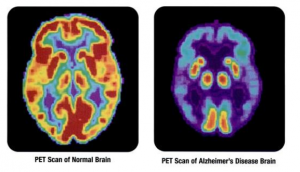
A third recent development that may help with early identification of AD patients focuses on the use of an artificial intelligence (AI) algorithm to track small changes in brain metabolism detected via PET imaging. In a 1,000-patient study, researchers showed that the algorithm was able to predict patients that progressed to AD with 82% specificity and 100% sensitivity by an average of 75.8 months earlier than they could be discerned by radiologists reading the same scans. The AI algorithm was trained on 90% of the 2,100 images per patient collected from the Alzheimer’s Disease Neuroimaging Initiative, and then tested on the remaining 10% of scans plus independent exams from 40 patients not previously studied. These findings need to be validated with a larger independent dataset and prospective study, but suggest that this method might be used along with other biochemical and imaging diagnostics to help achieve an AD diagnosis at an earlier stage of disease.
PET imaging is unlikely to ever be widely used as a diagnostic screen for Alzheimer’s disease, due to its high cost, limited availability, and use of radiation. However, it will be interesting to see if a similar AI-based approach could be used with lower-cost, more available MRI scans to improve AD diagnosis.