 The Bionest team attended the American Academy of Neurology (AAN) in early May, where we heard exciting data presented in a number of neurological conditions. Among the highlights of this year’s conference:
The Bionest team attended the American Academy of Neurology (AAN) in early May, where we heard exciting data presented in a number of neurological conditions. Among the highlights of this year’s conference:
Transformative Success in Spinal Muscular Atrophy
Spinal muscular atrophy (SMA) was a hot topic at AAN, with presentations of positive clinical data on both Avexis/Novartis’s gene therapy Zolgensma, and PTC Therapeutics/Roche’s oral survival motor neuron (SMN) splicing modifier risdiplam. Avexis/Novartis presented data from three studies, including the first interim data from the STRONG study of Zolgensma. Given as a single, one-time dose via spinal injection, Zolgensma has been shown to provide prolonged survival, rapid motor function improvement, and developmental milestone achievements that patients never experience if left untreated. Clinical investigators are calling Zolgensma “transformative” for SMA, especially in newly diagnosed patients. The great benefit of this treatment for SMA was rewarded with a U.S. Food and Drug Administration approval on May 24 for intravenous use of the drug in patients under two years old.
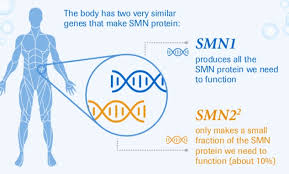 PTC/Roche presented similarly positive data for risdiplam, which works by helping the SMN2 gene produce more functional SMN protein, which is required for proper muscle function. Data from the FIREFISH and SUNFISH trials showed that treated infants achieved key motor milestones after one year of therapy. PTC/Roche plan to seek FDA approval later this year.
PTC/Roche presented similarly positive data for risdiplam, which works by helping the SMN2 gene produce more functional SMN protein, which is required for proper muscle function. Data from the FIREFISH and SUNFISH trials showed that treated infants achieved key motor milestones after one year of therapy. PTC/Roche plan to seek FDA approval later this year.
Both Zolgensma and risdiplam are expected to provide serious competition for Biogen’s Spinraza, which until now has had the SMA treatment market to itself. Novartis has priced Zolgensma at $2.1 million for a one-time treatment, paid via outcomes-based installments over five years. While Zolgensma is extremely expensive (although priced considerably lower than some speculated it would be prior to its approval), pricing watchdog ICER has confirmed that the price falls within the upper range of the cost-effectiveness scale. Moreover — assuming the one-time treatment’s benefits are sustained long-term — Zolgensma’s price is approximately half of the 10-year cost for Spinraza, which must be used for life. Risdiplam appears to work at least as well as Spinraza, while providing the advantages of an oral therapy versus spinal injection. Additionally, risdiplam’s mechanism of action means that it may be efficacious in older SMA patients versus Zolgensma, which shows the greatest benefit in newly diagnosed, pre-symptomatic infants.
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease affecting nerve cells in the brain and spinal cord that control muscles throughout the body. While four drugs are currently approved to treat the disease, better therapies are needed, and there is no cure.
 At AAN, Cytokinetics/Astellas announced results of the FORTITUDE study of reldesemtiv, which is designed to alleviate muscle weakness and fatigue in ALS. Although the trial failed to meet its primary efficacy endpoint at 12 weeks, Cytokinetics said that a pooled analysis of all reldesemtiv treatment groups showed clinically meaningful benefits emerging over time, prompting the company to plan for a Phase 3 study.
At AAN, Cytokinetics/Astellas announced results of the FORTITUDE study of reldesemtiv, which is designed to alleviate muscle weakness and fatigue in ALS. Although the trial failed to meet its primary efficacy endpoint at 12 weeks, Cytokinetics said that a pooled analysis of all reldesemtiv treatment groups showed clinically meaningful benefits emerging over time, prompting the company to plan for a Phase 3 study.
Biogen presented Phase 1 data on its ALS drug candidate, tofersen, which was licensed from Ionis Pharmaceuticals. Data showed tofersen is well-tolerated and suggested the drug could slow disease progression in people with a mutant SOD1 gene. SOD1 mutations cause the expression of a misfolded protein that is toxic to nerve cells, and tofersen is an antisense oligonucleotide designed to bind SOD1 mRNA, thus reducing production of the toxic protein. SOD1 mutations are a known cause of the familial form of ALS, and represent about 2% of all ALS cases. Biogen is now advancing tofersen into pivotal trials aimed at showing that the drug works in larger groups of patients, over longer periods of time.
Stay tuned for a second AAN report later this week.