Back in January, we noted our expectations that the application of digital technologies to healthcare would be an area of substantial innovation and growth in 2021. Digital innovations are beginning to make notable progress as tools to facilitate the better tracking of disease symptoms and patient responses to treatment. They are also helping clinical trial recruitment by allowing investigators to reduce the number of in-person visits by trial participants, thus lowering potential hurdles that may discourage patients from enrolling in a study.
Conditions like Parkinson’s disease (PD) are particularly benefitting from digital innovations, especially from wearable devices. PD is a highly variable condition, where symptoms differ from person to person and evolve over time as the disease progresses. As a result, treatment regimens are highly personalized. The challenges that many patients and neurologists face is that infrequent doctor visits provide only a snapshot of a patient’s status and response to medications, and patient self-reporting of symptoms may not paint a complete or accurate picture as it is highly subjective. Thus, devices that can be worn to continuously monitor symptoms have the potential to greatly assist physicians in providing better care.
Several wearables have either been created or are in development to monitor PD symptoms. Common devices such as smartphones equipped with inertial sensors, insoles capable of measuring gait and fitness, and specialized products like Global Kinetics’ Personal Kinetigraph, a wrist-worn device that collects data on movement and tremor and provides patients with medication reminders, can contribute to monitoring patients’ symptoms and providing them with disease management assistance. An emerging idea is to use familiar, unobtrusive tools, like smart watches, to continuously monitor disease progression from an early stage. But to date, few of these wearables have received regulatory approval and are commercially available.
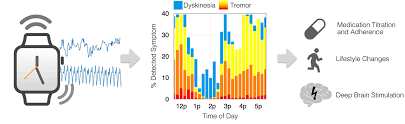
In February, researchers from Apple and their medical collaborators published results in Science Translational Medicine from two clinical studies of a new system called the Motor Fluctuation Monitor for PD (MM4PD). The MM4PD uses accelerometer and gyroscopic data from the Apple Watch to detect the presence of resting tremor or dyskinesia, a type of involuntary movement that is often a side-effect of PD medications. Unlike some other digital monitoring tools, which require patients to manually input data from their wearable into a system for analysis, the MM4PD requires no patient input beyond wearing the watch.
In an initial pilot study, the researchers developed algorithms based on motor symptom data from 118 individuals with PD. The data from each subject’s Apple Watch was matched to the “gold standard” scoring system for measuring motor symptoms of PD. The exercise involved a team of three movement disorder specialists who rated video recordings of the trial subjects that were time-aligned with the smart watch data. In a second study, researchers tracked 225 people with PD for up to six months and used their watches’ data to create PD symptom profiles. As a control group, the researchers also monitored 171 older individuals without PD for up to 12 months. The profiles were then evaluated and curated by clinicians to determine if the MM4PD could identify motor responses to changes in treatment and determine whether it could be used to support clinical decision-making.
The authors concluded that the MM4PD system can help spot symptoms missed in regular care and can identify changes in symptoms after subjects undergo surgery for deep brain stimulation. Study results also suggest that the tool can help identify individuals who are non-adherent to medications and those who might benefit from adjustment of their treatment regimen. The MM4PD system still faces a few limitations, however, as the tool focuses only on two of the motor symptoms most common in PD and uses the wrist as a single observation point, which may not capture symptoms in other parts of the body.
Apple has not yet announced plans to create a commercially available MM4PD system leveraging the Apple Watch for PD monitoring on a broader scale, but if they do, it won’t be the company’s first commercial digital health offering. They already commercialize an Apple Watch app that can detect heart rhythm changes related to atrial fibrillation, a common type of arrhythmia that can lead to feelings of weakness and an increased risk of stroke, and another one designed to help improve sleep in patients with PTSD suffering from recurring nightmares, available by prescription. The MM4PD system may also be applicable beyond PD, to other neurologic conditions with associated measurable motor system alterations, like Alzheimer’s disease and other tremor-related disorders.